Bethesda, MD — A new peer-reviewed paper by Dr. Zaher Nahle, Senior Scientific Advisor to the Center for a Humane Economy and Animal Wellness Action, is published in Frontiers in Medical Technology, offering a novel and comprehensive analysis of how animal testing became deeply embedded in drug development and biomedical research—and how new national policies promoting alternative methods and innovations in science are finally breaking the logjam and producing a paradigm shift in research and development in the United States and perhaps also around the world.
In the article, “Path Dependency and the Rescuing of the Biomedical Research Enterprise,” Dr. Nahle applies the social sciences concept of path dependency to trace how regulatory decisions, dating back to 1938, locked drug development into animal-centric testing, despite mounting evidence that it poorly predicts human outcomes. The paper documents how this legacy framework has contributed to a 92% failure rate in translating drugs from preclinical studies into approved therapies.
“Animal testing didn’t dominate because it was the best science—it dominated because early regulatory decisions locked it in,” wrote Dr. Nahle. “What we are witnessing now is the deliberate dismantling of that path dependency and the emergence of a more predictive, humane, and efficient biomedical research enterprise.” — Path Dependency and the Rescuing of the Biomedical Research Enterprise
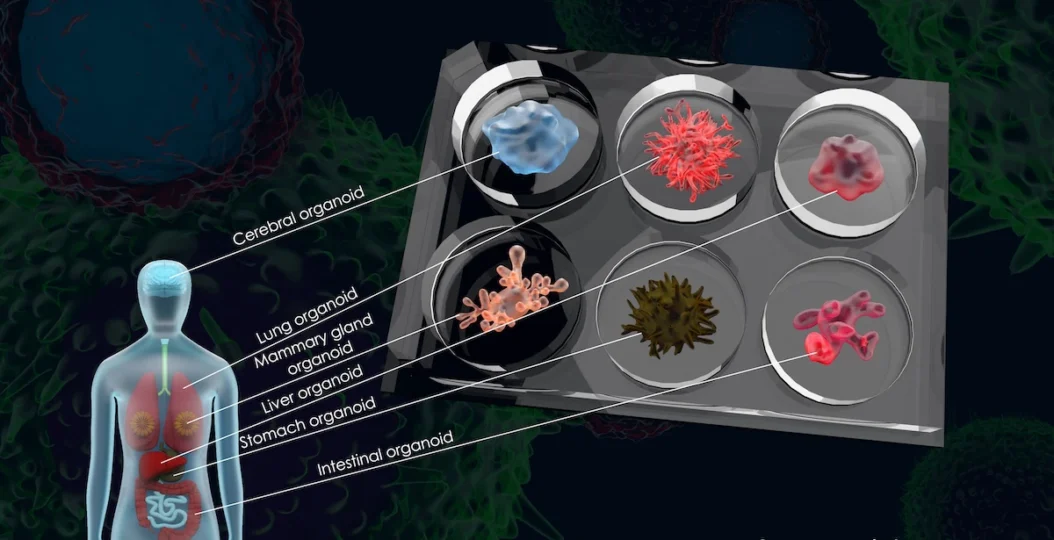
The paper highlights the growing role of human-relevant New Approach Methods (NAMs)—including organoids, organ-on-chip platforms, and advanced computational models—and examines how recent federal actions have accelerated their adoption. Among the key developments discussed is the FDA Modernization Act 2.0, enacted in 2022, which removed the statutory requirement for animal testing and allowed regulators greater flexibility to rely on non-animal, human-based science.
“Animal testing became the norm in the realms of drug development and biomedical research because of regulatory capture, profit motives, and habit rather than its scientific merits,” said Wayne Pacelle, president of Animal Wellness Action and the Center for a Humane Economy. “With the enactment of the FDA Modernization Act 2.0 as Dr. Zaher Nahle explains in his important new paper, the global scientific community is now expected to upend the old construct and embrace 21st-century science that is more predictive, more humane, and more relevant to patients.”
While acknowledging recent progress at agencies including the FDA, NIH, EPA, and CDC, the paper also cautions against serious missteps like treating non-animal methods as mere complements to animal testing, arguing that such framing risks advancing the principles of the 3Rs and perpetuating inefficiencies. The work methodically enumerates the steps needed to capitalize on the forward momentum and candidly assesses opportunities and challenges. It also highlights the still-required contributions from all stakeholders across the discovery research ecosystem to achieve a durable, long-lasting transformation.
The study is published as Congress continues to debate next steps in implementing these reforms.
Animal Wellness Action and the Center for a Humane Economy are urging the U.S. House of Representatives to bring the FDA Modernization Act 3.0 to the floor for a vote, ensuring full and timely implementation of Congress’s intent to modernize drug development and reduce reliance on animal testing.
You can download the open-access article from Frontiers in Medical Technology using this link. The abstract is provided below:
In 2025, three U.S. agencies within the Department of Health and Human Services (FDA, NIH, CDC) alongside EPA and the Departments of the Navy and Veterans Affairs, began substituting animal testing applications with reliable, human-relevant methods. The impact of this shift in public policy taking place at agencies historically bullish on animal testing is still reverberating in the United States and around the world. Here, we examine the circumstances that enabled such momentous reforms, including the role of the FDA Modernization Act 2.0 in advancing new alternative methods, collectively referred to as NAMs. We explain how animal testing, despite its poor value in predicting the safety and efficacy of drugs in humans, came to dominate drug discovery, basic sciences, and environmental toxicity assessments since the inception of the Federal Food, Drug, and Cosmetic Act (Federal FD&C Act) in 1938. Specifically, we identify critical junctures, including catastrophic government decisions, that made the overall research enterprise acutely dependent on animals, leading to the existing predicament—an indefensible 92% failure rate in translating drugs from preclinical studies to actual therapies. Notably, our analysis chronicles events through the lens of “path dependency,” a social sciences phenomenon that occurs when faulty past decisions lock-in future action. Finally, we recognize that our narrative is a departure from the medical establishment account or the talking points of powerful interest groups, including some in the academic elite who continue to shore up animal-centric paradigms in drug development for reasons we also outline.

